A pH meter is an instrument used to measure acidity or alkalinity of a solution - also know as pH. pH is the unit of measure
that describes the degree of acidity or alkalinity. It is measured on a scale of 0 to 14.
The quantitative information provided by the pH measurement expresses the degree of the activity of an acid or base in terms of hydrogen ion activity.
The pH value of a substance is directly related to the ratio of the hydrogen ion [H+] and the hydroxyl ion [OH-] concentrations. If the H+ concentration
is greater than OH-, the material is acidic; i.e., the pH measurement is less than 7. If the OH- concentration is greater than H+, the material is basic,
with a pH value greater than 7. If equal amounts of H+ and OH- ions are present, the material is neutral, with a pH of 7.
Acids and bases have free hydrogen and hydroxyl ions, respectively.
The relationship between hydrogen ions and hydroxyl ions in a given solution is constant for a given set of conditions, either one can be
determined by knowing the other.

pH Measurement
A rough indication of pH can be obtained using pH papers or indicators, which change color as the pH level varies.
These indicators have limitations on their accuracy, and can be difficult to interpret correctly in colored or murky samples.
What does the term "pH" mean?
The term pH is derived from “p,” the mathematical symbol for negative logarithm, and “H,” the chemical symbol for Hydrogen.
More accurate pH measurements are obtained with a electronic pH meter. A pH measurement system consists of three parts: a pH measuring electrode,
a reference electrode, and a high input impedance meter. The pH electrode can be thought of as a battery, with a voltage that varies with
the pH of the measured solution. The pH measuring electrode is a hydrogen ion sensitive glass bulb, with a millivolt output that varies
with the changes in the relative hydrogen ion concentration inside and outside of the bulb. The reference electrode output does not vary
with the activity of the hydrogen ion. The pH electrode has very high internal resistance, making the voltage change with pH difficult to
measure. The input impedance of the pH meter and leakage resistances are therefore important factors. The pH meter is basically a high
impedance amplifier that accurately measures the minute electrode voltages and displays the results directly in pH units on either an analog
or digital display. In some cases, voltages can also be read for special applications or for use with ion-selective or Oxidation-Reduction
Potential (ORP) electrodes.
pH Electrodes
pH electrode technology has not changed much in the past 50 to 60 years. With all the technological advancements of the last 30 to 40 years, pH
electrode manufacturing remains an art. The special glass body of the electrode is blown to its configuration by glass blowers. Not a terribly
advanced nor “high tech” process but a very critical and important step in the electrode manufacturing. In fact, the thickness of the glass
determines its resistance and affects its output.
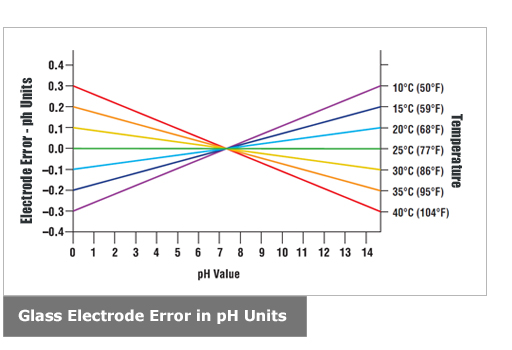
Temperature Compensation
Temperature compensation is contained within the instrument, because pH electrodes and measurements are temperature sensitive.
The temperature compensation may be either manual or automatic. With manual compensation, a separate temperature measurement is required,
and the pH meter manual compensation control can be set with the approximate temperature value. With automatic temperature compensation (ATC),
the signal from a separate temperature probe is fed into the pH meter, so that it can accurately determine pH value of the sample at that temperature.
Buffer Solutions
Buffers are solutions that have constant pH values and the ability to resist changes in that pH level. They are used to calibrate pH
measurement systems (electrode and meter). There can be small differences between the output of one electrode and another, as well as changes
in the output of electrodes over time. Therefore, the system must be periodically calibrated. Buffers are available with a wide range of pH values,
and they come in both premixed liquid form or as convenient dry powder capsules. Most pH meters require calibration at several specific pH values.
One calibration is usually performed near the isopotential point (the signal produced by an electrode at pH 7 is 0 mV at 25°C), and a second is
typically performed at either pH 4 or pH 10. It is best to select a buffer as close as possible to the actual pH value of the sample to be measured.


Choose the right pH meter
 Wireless pH Meters
Wireless pH Meters
Bluetooth wireless transmitters that connect with smart phones or tables to log and monitor pH measurements.
These transmitters measure different sensor inputs,
including but not limited to pH, RTD, relative humidity. The data transmission is performed via Bluetooth wireless technology to a smart phone or tablet
with the app installed. The app will allow the smart phone to pair and set up multiple transmitters.
 Portable pH Meters
Portable pH Meters
These pH meters offer full functionality in a portable size for use in the lab or field. Features available include RS232
output, Data Hold, °C or °F selectable, auto shutoff, overload indication and automatic or manual temperature compensation, and more.
 Benchtop pH Meters
Benchtop pH Meters
Benchtop pH meters are usually ideal for laboratory, industrial, and manufacturing applications. Models offer mV, ion and temperature measurement and
range from economical to high-end for low-tolerance measurement needs.
 pH Electrodes
pH Electrodes
pH electrodes are available in a variety of styles for both laboratory and industrial applications. No matter their status, they
are all composed of glass and are therefore subject to breakage. Electrodes are designed to measure mostly aqueous media.
They are not designed to be used in solvents, such as CCI4, which does not have any free hydrogen ions.
Frequently Asked Questions
Effects of Temperature on pH
Temperatures Above 25°C: temperature compensation lowers high pH and raises low pH, resulting in value closer to neutral.
Temperatures Below 25°C: temperature compensation raises high pH (more basic) and lowers low pH (more acidic), resulting
in values further away from neutral. Whether or not temperature compensation needs be used is a matter of the needed
pH accuracy. For example, if the accuracy requirement is ±0.1 pH, at a pH of 6 and a temperature of 45°C (113°F), the error
is 0.06, well within the accuracy requirements. On the other hand, with the same ±0.1 pH accuracy requirement, operating at pH 10 and 55°C (131°F)
would give an error of 0.27 pH and compensation should be used.
When compensation is required, it can be done in one of two ways. If the temperature fluctuates, then an automatic compensator should be used. If the
temperature is constant within several degrees C, then a manual compensator can be used. If no compensator is needed, a fixed resistor can be
installed across the temperature compensator terminals.
Any of the above devices–automatic compensation, manual compensation or fixed resistor–operate as a function of the pH meter’s electronic circuit. As such,
information and parts should be obtained from the meter manufacturer. If automatic compensators are used, they should always be at the same location
as the pH electrode. When electrodes are calibrated in buffer, the temperature compensator also should be in the buffer. In a similar way, a manual
temperature compensator should be adjusted to reflect the temperature to which the pH electrode is exposed during both calibration and operation.
How to calibrate a pH Meter?
Here is a general method for most pH meters. Some pH meters require slightly different techniques. Please read the instructions for
their particular procedures.
- The temperature setting on the meter must correspond to the temperature of the buffers used, or an automatic temperature
compensator must be employed.
- Turn pH meter to “pH” or “ATC” if automatic temperature compensation is used.
- Place clean electrode into fresh, room temperature pH 7.00 buffer.
- Adjust the pH reading to exactly 7.00 using the ZERO OFFSET,STANDARDIZED or SET knob.
- Rinse the electrode with distilled or deionized water. (This would be the procedure for a one-point calibration. Continue
through step 8 for a two-point calibration.)
- Place electrode into the second buffer, either pH 4.00 or pH 10.00.
- Adjust the pH reading to display the correct value using the SLOPE, CALIBRATE, or GAIN controls (coarse adjust).
- Adjust the pH reading to read the correct value using the SLOPE knob (fine adjust).
Challenges of pH Measurement Applications
No pH complications are created equal. The list below illustrates the types of problems that you can expect when measuring pH and how to handle them.
- Instrumentation is frequently the source of disturbance for pH systems, through repeatability error, measurement noise, or valve hysteresis.
- In-line pH loops will oscillate, regardless of controller modes and tuning, if setpoints are on the steep parts of the titration curves.
- pH electrode submersion assemblies with unencapsulated terminations below the liquid surface will eventually have wet terminations.
- Reagent control valves that are not close-coupled to the injection point on in-line systems will cause reagent delivery delays large
enough to describe the tools of your trade n words that may seem foreign.
- You need either a flow meter or a seer to diagnose reagent delivery problems.
- Flow feedforward signals should be multiplied by pH controller outputs and employed to operate reagent valves directly or to establish reagent
flow control setpoints.
- Transportation delays to pH electrodes in analyzer houses will exceed mixing deadlines - such that increasing comfort in checking the
electrodes is offset by decreasing comfort in checking trend recordings.
- Injection electrodes should be preferred to sample holder assemblies whenever possible to reduce maintenance problems and improve
response times - but not all injection electrodes are created equal.
- Large tanks are fine if you don't have to control them; use the volume upstream to reduce reagent consumption or downstream to reduce
control error. If you can't make-up your mind where to use one, put it downstream.
- Install one or three but never two electrodes for a pH measurement.
↓ View this page in another language or region ↓
 CLOSE
CLOSE
















 Medidor de pH
Medidor de pH pH meter
pH meter pH meter
pH meter pH meter
pH meter pH meter
pH meter pH meter
pH meter pH计
pH计 pH meter
pH meter
 pH meter
pH meter
 pH meter
pH meter
 pH meter
pH meter
 pH meter
pH meter
 pH meter
pH meter