|
|
 |
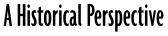
Force & Its Effects
Force is a quantity capable of changing the size, shape, or motion of an object. It is a vector quantity and, as such, it has both direction and magnitude. In the SI system, the magnitude of a force is measured in units called newtons, and in pounds in the British/American system. If a body is in motion, the energy of that motion can be quantified as the momentum of the object, the product of its mass and its velocity. If a body is free to move, the action of a force will change the velocity of the body.
There are four basic forces in nature: gravitational, magnetic, strong nuclear, and weak nuclear forces. The weakest of the four is the gravitational force. It is also the easiest to observe, because it acts on all matter and it is always attractive, while having an infinite range. Its attraction decreases with distance, but is always measurable. Therefore, positional "equilibrium" of a body can only be achieved when gravitational pull is balanced by another force, such as the upward force exerted on our feet by the earth's surface.
 |
| Figure 1-3: Atmospheric Reference Gauge |
Pressure is the ratio between a force acting on a surface and the area of that surface. Pressure is measured in units of force divided by area: pounds per square inch (psi) or, in the SI system, newtons per square meter, or pascals. When an external stress (pressure) is applied to an object with the intent to cause a reduction in its volume, this process is called compression. Most liquids and solids are practically incompressible, while gases are not.
The First Gas Law, called Boyle's law, states that the pressure and volume of a gas are inversely proportional to one another: PV = k, where P is pressure, V is volume and k is a constant of proportionality. The Second Gas Law, Charles' Law, states that the volume of an enclosed gas is directly proportional to its temperature: V = kT, where T is its absolute temperature. And, according to the Third Gas Law, the pressure of a gas is directly proportional to its absolute temperature: P = kT.
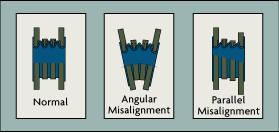 |
| Figure 1-4: Flexible Load-Cell Connections |
Combining these three relationships yields the ideal gas law: PV = kT. This approximate relationship holds true for many gases at relatively low pressures (not too close to the point where liquification occurs) and high temperatures (not too close to the point where condensation is imminent).
|

